Looking for a non-surgical option to open and lift your eyes?
Looking for a non-surgical option to open and lift your eyes? We’ve got the simple, once-a-day painless fix for those droopy upper eyelids. For patients who are not ready for surgical eyelid correction. Youtherapy is excited to offer their patients Upneeq—an FDA-approved eye drop that helps give your eyes a more open, brighter, and beautiful appearance. With the introduction of Upneeq to the armamentarium, we now have a non-surgical method to open up those droopy eyelids using a once daily eye drop. It sounds hard to believe, but it’s true!
What are droopy eyelids (Blepharoptosis)?
Blepharoptosis is a medical term for droopy eyes. People with this condition appear to be tired, and their eyes look smaller. Droopy eyelids can also interfere with normal eye function. There are numerous causes for this condition. Some of them include genetics, age, and previous eye surgery. Even having a Botox injected into your forehead can lead to droopy eyelids. Blepharoptosis can be congenital (meaning that it is present from birth) or acquired, meaning that it develops later. Upneeq eye drops are not designed to treat congenital droopy eyes.


Frequently Asked Questions
What treatments are available for droopy eyelids?
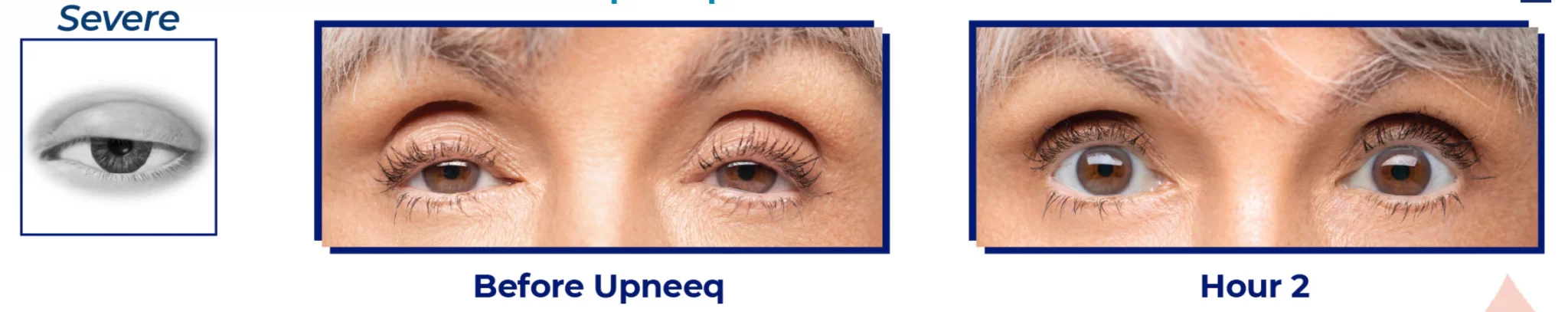
Until Upneeq was available, the majority of people suffering from droopy eyelids went for eyelid lift surgery to correct. Upneeq is a non-surgical alternative approved by the FDA to specifically treat acquired Blepharoptosis in adults.
How long does Upneeq last?
According to clinical studies, the effects of each daily dose of Upneeq lasts about 8 hours, giving a temporary eyelid lift.
Is Upneeq approved by the FDA?
Yes, Upneeq is the first FDA-approved prescription eye drop treatment for the droopy upper eyelid. Clinical trials proved that Upneeq is safe and effective for droopy eyelids treatment in adults.
Upneeq eye drops vs. eyelid lift surgery?
Upneeq eye drops are a temporary alternative to eyelid surgery. They offer temporary and mild improvement for people suffering from acquired Blepharoptosis. As such, they are not substitute for eyelid surgery. Eyelid surgery offers permanent results and can help people suffering from both acquired and genetic Blepharoptosis.
Warnings and precautions
- Alpha-adrenergic agonists as a class may impact blood pressure. Advise UPNEEQ patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to seek medical care if their condition worsens.
- Use UPNEEQ with caution in patients with cerebral or coronary insufficiency or Sjogren’s syndrome. Advise patients to seek medical care if signs and symptoms of potentiation of vascular insufficiency develop.
- UPNEEQ may increase the risk of angle-closure glaucoma in patients with untreated narrow-angle glaucoma. Advise patients to seek immediate medical care if signs and symptoms of acute narrow-angle glaucoma develop.
- Patients should not touch the tip of the single patient-use container to their eye or to any surface in order to avoid eye injury or contamination of the solution.
Adverse Reactions
Common side effects that occurred in 1-5% of subjects treated with UPNEEQ were punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, eye irritation, and headache.
Drug Interactions
- Alpha-adrenergic agonists, as a class, may impact blood pressure. Caution in using drugs such as beta-blockers, anti-hypertensives, and/or cardiac glycosides is advised. Caution should also be exercised in patients receiving alpha-adrenergic receptor antagonists, such as in treating cardiovascular disease or benign prostatic hypertrophy.
- Caution is advised in patients taking monoamine oxidase inhibitors which can affect the metabolism and uptake of circulating amines.